The Pharmaceutical Industry is incredibly dynamic, with constant updates occurring all through various fields encompassing the industry. Pharmexon has gathered Regulatory Intelligence from September 3rd through October 22nd, in addition to other news, enabling you to become more knowledgeable about the most recent changes throughout the pharmaceutical industry.
The RUP Request Form has been Updated
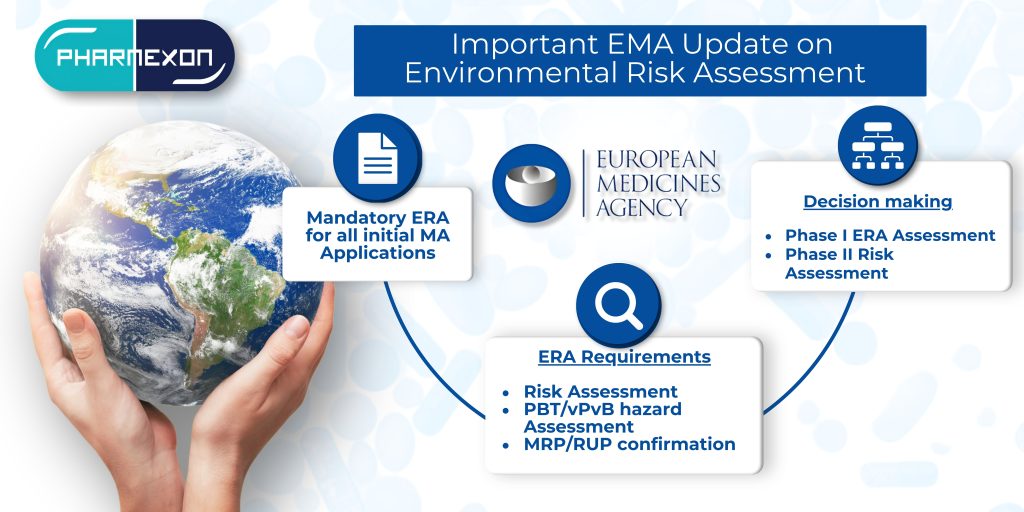
Reported from the CMDh, applicants are now requested to confirm before the start of an MRP/RUP that the ERA provided in Module 1.6 is compliant with the current guideline version. Until 31 March 2025, these requests can be submitted with the possibility to commit to a variation submission within 3 months after the end of an MRP/RUP in case the ERA provided in the above module is not in line with the current guideline version.
CMDh Update on their Q&A on Pharmacovigilance
This is specific is in terms of extrapolation of PSUSA outcomes to other products not within the scope of the single assessment (other FDCs/mono products) and for drug-drug interactions.
- The following active substances agreed by consensus in variation of marketing authorization are as follows:
- 5-fluorouracil (IV use) allopurinolamantadineamiodaroneamitriptyline, amitriptyline / amitriptylinoxide, amitriptylinoxideamitriptyline / perphenazinelevonorgestrel / ethinylestradiol, ethinylestradiol (combination pack)liothyronine macrogol 3350 combinations (oral use) valproic acid, sodium valproate, valproate pivoxil, valproate semisodium, valpriomide, valproate bismuth, calcium valproate, valproate magnesium
PRAC has Published Criteria to be Used for its Impact Research
They have done so under the remit of the PRAC Impact Strategy. The criteria are broken down into three categories – Public Health Importance of the Regulatory Action, Delivery of Decision Relevant Data, and Regulatory Follow-Up.
Under the Public Health Importance of the Regulatory Action, the criteria include the
- Nature and severity of the risk in target population
- Magnitude of the risk (absolute and relative) in target population
- Amount of public concern, and extent of regulatory intervention.
Focusing on the Delivery of Decision Relevant Data category, the criteria include questions such as
- Regulatory action is amendable to research generating impact relevant data?
- Suitable data sources and methodologies are available in several member states to allow generalisability of results?
- Does the study fill gaps in knowledge and understanding of RMM effectiveness?
- Does the study complement evidence from MAH-sponsored RMM effectiveness studies?
- Which further regulatory actions may be warranted?
CMDh CEP Variations:
Recommendations on how to submit CEP updates when nitrosamine impurities are involved has been provided. Suggestions are made for both situations:
- MA’s containing approved revision of CEP before the inclusion of the nitrosamine impurity
- Two type IB variations are offered as condition number two is not fulfilled: one variation for the inclusion and one for the deletion of the impurity.
- MA’s containing the approved revision of CEP with included nitrosamine impurity
- The variation guideline mandates a type IB for the registration of the latest version of the CEP.
CHMP Elects New Chair
Bruno Sepodes began his three-year mandate September 21st, succeeding Dr. Harald Enzmann, who completed the maximum number of allowed CHMP chair terms.
EMA has recommended 8 new medicines to be approved
Elahere, Hetronifly, Hympavzi, Penbraya, Theralugand, Afqlir, Opuviz, and Pomalidomide Teva have all been recommended to be approved for marketing.
CHMP gave positive opinions for Spikevax and Comirnaty
The CHMP gave positive opinions to update the composition of the mRNA vaccine Spikevax, to target the SARS-CoV-2 JN.1 variant of the virus that causes COVID-19, and Comirnaty for the KP.2 subvariant. The revision of these vaccines is in line with the recommendations issued by EMA’s Emergency Task Force to update COVID-19 vaccines to target the SARS-CoV-2 variants for the 2024/2025 vaccination campaign.
CMDh Contact Points have been Updated
The CMDh contact points excel has been updated. Make sure you have the latest version.
CMDh October Meeting
CMDh Q&A documents will be updated, in addition to important information about access to IRIS being announced (due to the transition of post-authorization procedure management to the IRIS platform in January, 2025). Roles will need to be requested earlier for products where you are the contact person.
ICH M14 Guideline
Comments on the pharmacoepidemiologic studies have been published.