Knowing that the healthcare industry is constantly growing and evolving, we knew that we had to actively engage in our client’s key developments to understand their needs 🚀 .
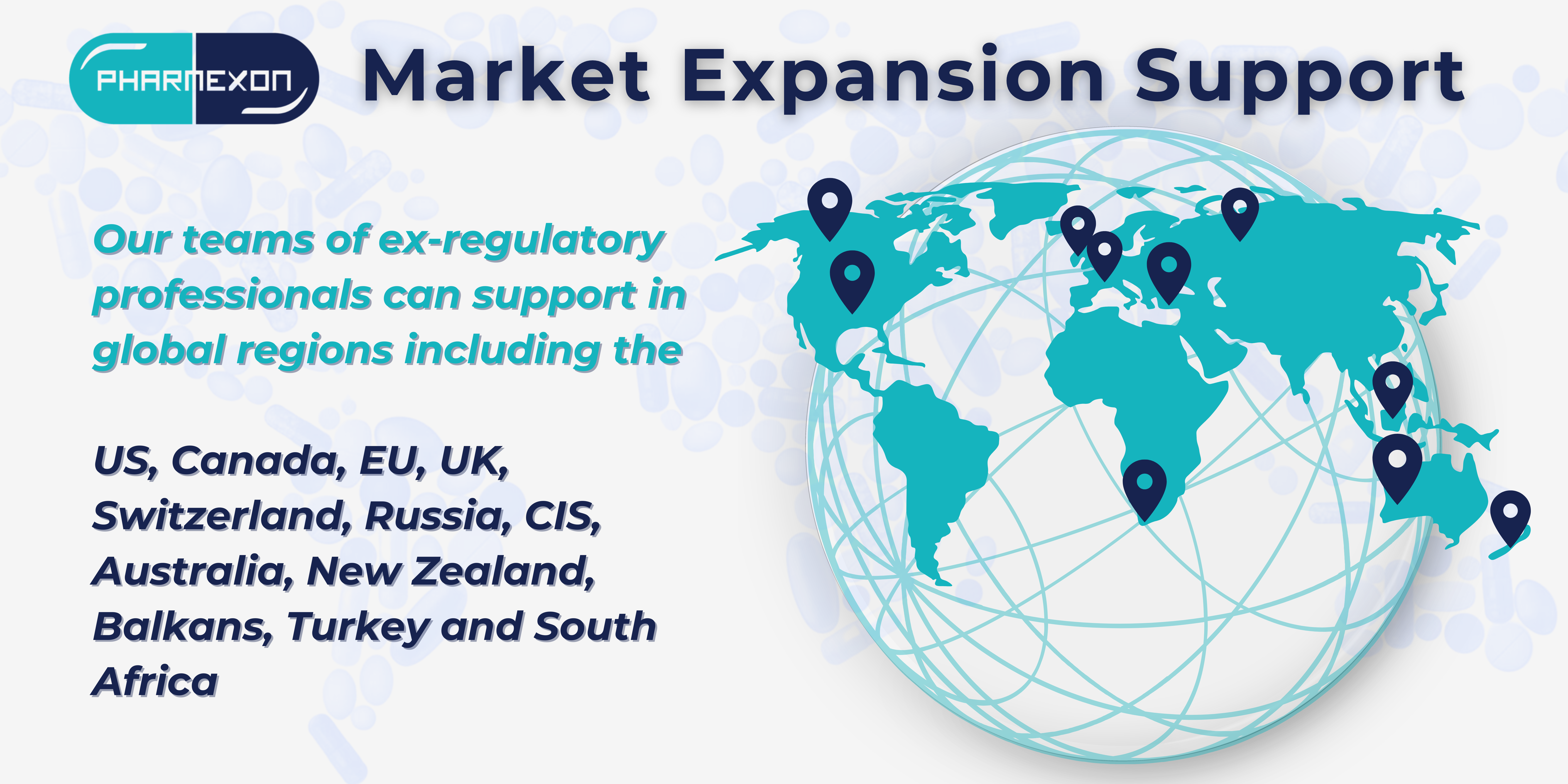
For years we have been helping companies to expand to global markets sharing a deep understanding of stringent regulations 🔒 . As a dedicated partner to several companies located outside of the European Union, one of our current activities is assisting our clients to successfully expand into the EU market.
Entering the intricate landscape of the EU market can be complicated, and we can facilitate the transition by offering comprehensive services in regulatory strategy, country selection, and legal framework identification.
Our expert team creates a tailored regulatory strategy while taking into account the specific needs and nuances of each of our client’s products.
Our great strength lies in the teams of ex-regulatory professionals who can support in 🌐 global regions including the US, Canada, EU, UK, Switzerland, Russia, CIS, Australia, New Zealand, Balkans, Turkey, and South Africa.
If you require assistance in market expansion into the EU or other global markets, Pharmexon is glad to support you.