This fall a significant EMA update of guideline on Environmental Risk Assessment (ERA) for Medicinal Products for human use took effect!
🔍 Key Highlights:
- Scope of Application:
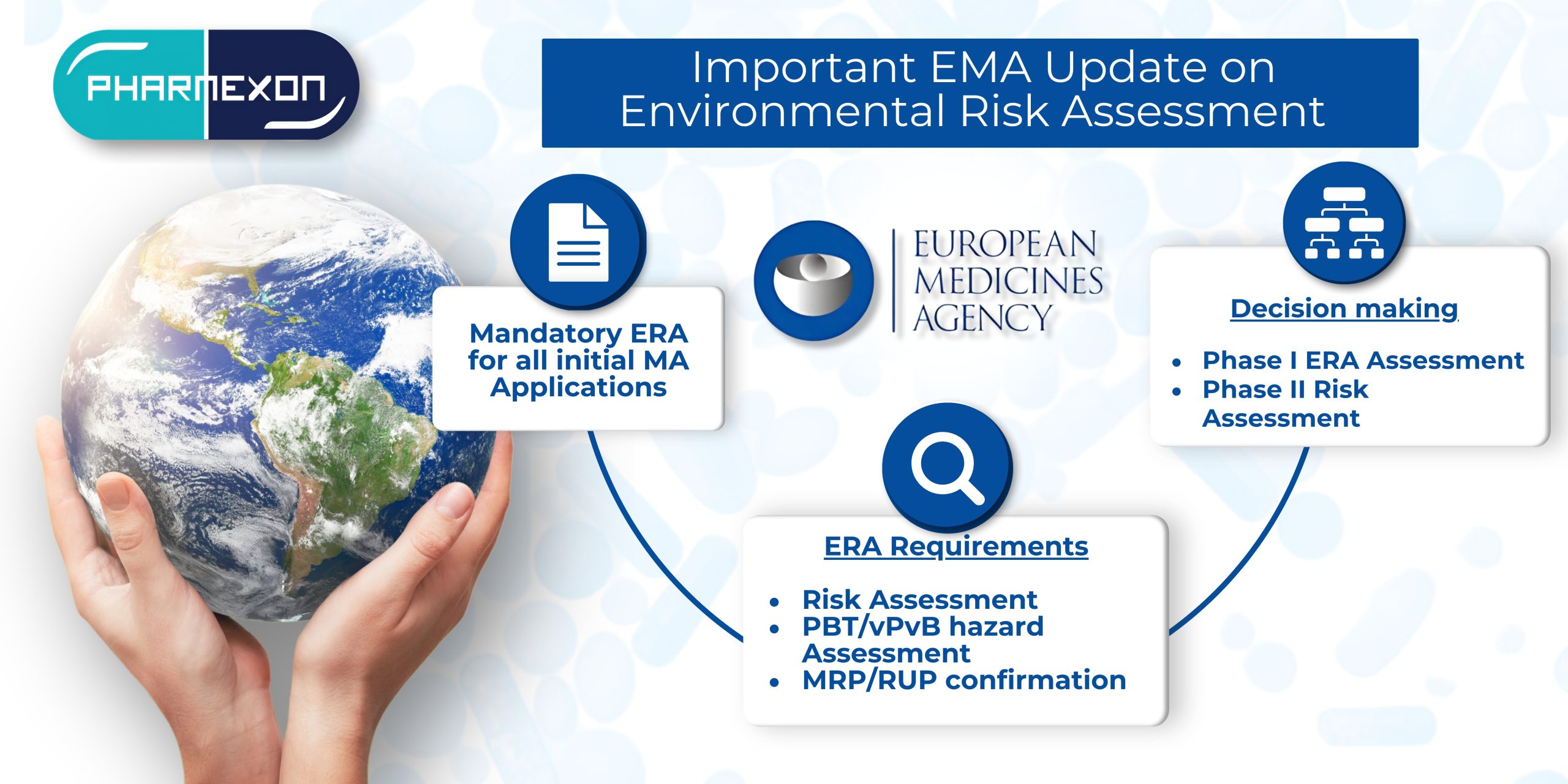
- An ERA is mandatory for all initial Marketing Authorization Applications and certain post-authorization applications (e.g., extension applications), regardless of the legal basis.
- ERA Requirements:
- The risk assessment evaluates the possibility of environmental impact, considering exposure levels and ecotoxicity.
- Applicants are requested to confirm before the start of an MRP/RUP that the ERA provided in Module 1.6 is in line with the current version of the guideline. For MRP/RUP requests submitted until 31 March 2025, it is possible to provide a commitment to submit a variation within 3 months after end of MRP/RUP in case the ERA provided in Module 1.6 is not in line with the current version of the Guideline.
- Applications in accordance with Article 10 of Directive 2001/83/EC may, in certain cases, rely on ERA previously submitted for the same active substance.
- Decision tree
- To identify if our clients’ products are fit for Phase I ERA, we now follow the decision tree presented in the updated guideline. The outcome of Phase I may be that the risk assessment is sufficient, or that a Phase II risk assessment is required.
- Our expert team will pre-assess the product based on the criteria outlined in the guideline and we will inform you about the likelihood of a Phase I assessment being accepted.
🌍 Our Commitment: We are here to support you in navigating these new requirements, preparing ERA reports, and addressing any deficiencies. If you need expert assistance in Environmental Risk Assessment and Toxicology, please reach out to us at BDinquiries@pharmexon.com.