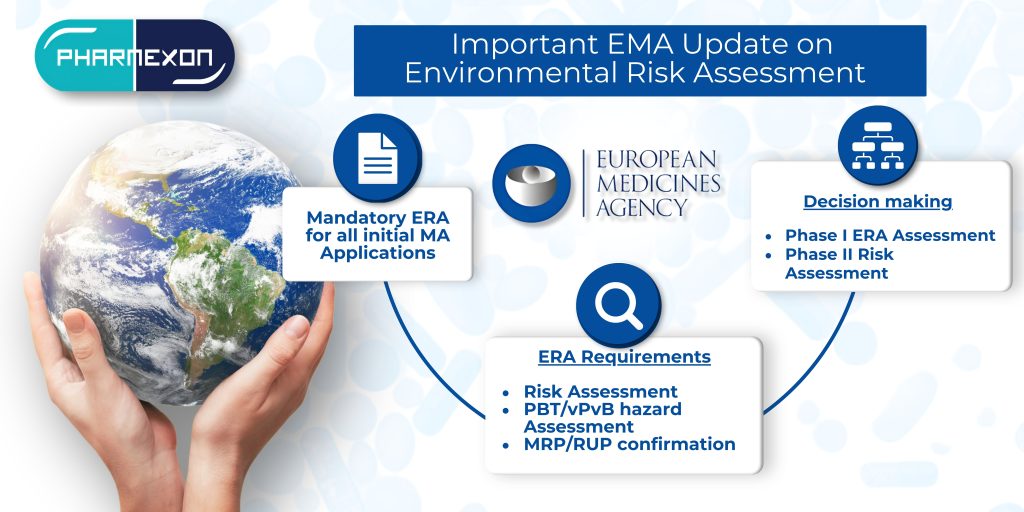
As the regulatory landscape evolves in 2025, pharmaceutical companies face both exciting opportunities and complex challenges. With the introduction of new tools and frameworks by the EMA and HMA, staying ahead of these changes is not just about compliance—it’s about ensuring efficiency in bringing products to market.
The key to success lies in adopting an informed and agile approach, making proactive planning, and leveraging the right expertise. Let’s explore the regulatory updates shaping 2025 and how Pharmexon can support you through this journey.
Key Compliance Challenges for Pharma in 2025
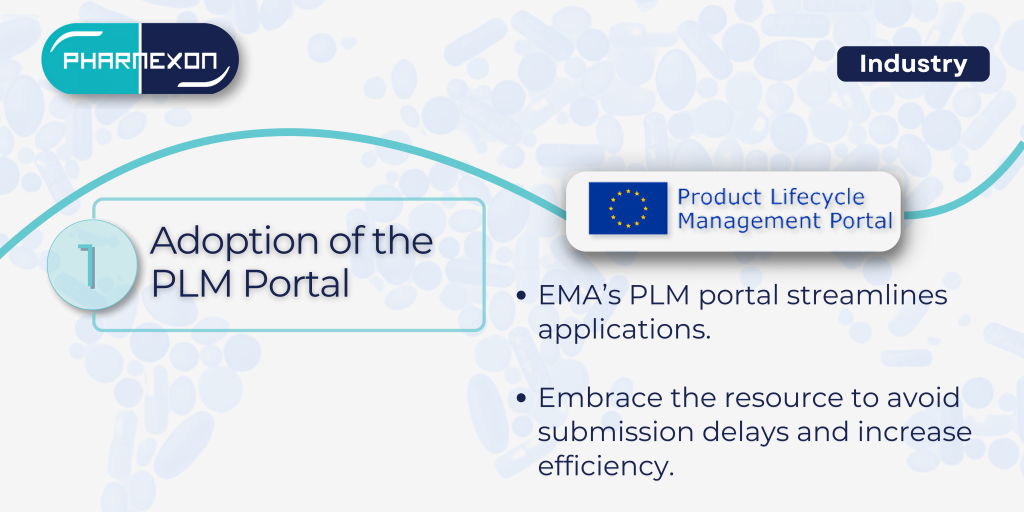
1. Adoption of the PLM Portal Application Form
The EMA’s new PLM portal is set to become a vital tool in managing applications. Its updated application forms are designed to streamline the process, but applicants must be well-versed in using this platform to avoid delays or errors in submissions. A strong focus on training and adapting internal processes will be essential to smooth integration.
2. Mastering the IRIS Portal
The IRIS portal, an essential platform for managing regulatory activities, is gaining prominence. To transition successfully to IRIS, applicants will need to master the portal’s technical aspects and adapt their workflows to meet its specific requirements. This shift aligns with EMA’s broader digital transformation efforts in regulatory affairs.

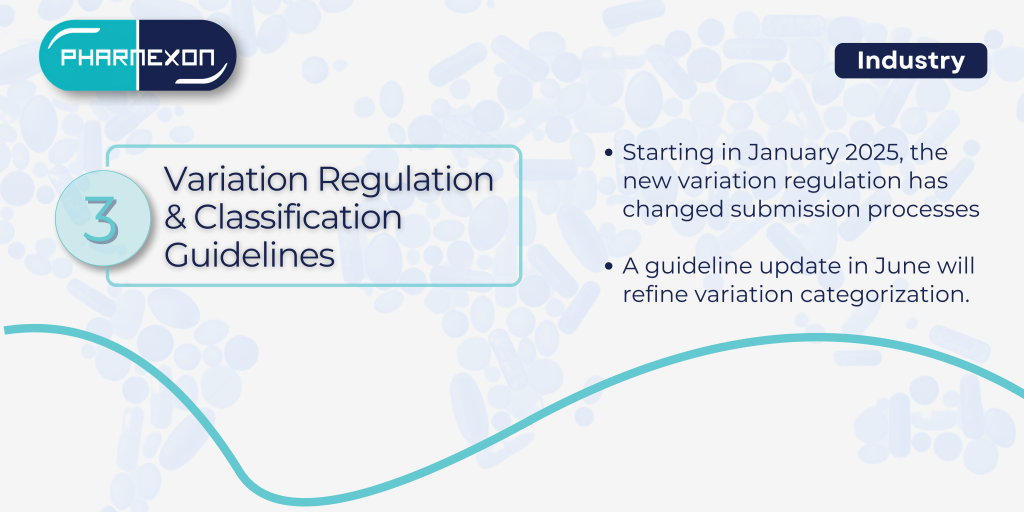
3. Variation Regulation and Classification Guidelines
The new variation regulation, effective January 2025, introduces significant changes in submission processes. Due to be released in June 2025, the classification guideline will further refine the categorization of variations. Navigating the intersection of these two updates will present challenges, especially for those managing diverse product portfolios.
4. Alignment with HMA and EMA Guidelines
To maintain regulatory compliance, companies will need to stay aligned with the ongoing updates from the HMA and EMA, which are working to harmonize regulatory practices across Europe. Keeping pace with these changes while ensuring operational efficiency will be crucial in 2025.
Pharmexon Strategic Solutions
To help manufacturers navigate the evolving regulatory landscape in 2025, Pharmexon offers strategic solutions integrated with our full range of services.
- Streamline Portal Management: With our Submission Management services, you don’t need to worry about mastering the PLM and IRIS portals. We handle all aspects of submission preparation and filing, allowing you to focus on product development without the technical complexities.
- Partner with Regulatory Experts: Our experienced consultants provide expert advice to interpret and implement the latest regulations. Through our Marketing Authorisation Applications support, we ensure you stay ahead of regulatory updates and deadlines, whether for national submissions, DCP, RUP/MRP, or CP.
- Proactive Planning: Plan for the phased implementation of regulations like variations and classification guidelines. Pharmexon’s Variation Classification & Management services help you align with these updates and stay compliant without disrupting your workflow.
By integrating strategic planning with our full suite of regulatory services, Pharmexon helps you navigate the 2025 challenges with ease and confidence. Our expert team ensures you stay ahead of regulatory changes and keeps your path to market smooth, efficient, and fully compliant. Let us manage the complexities, so you can focus on driving your product’s success.